Bond Order in a Molecule With 10 Valence Electrons
In the Lewis electron structures. Express your answer using two significant figures.

Hello My Name Is Bond Ionic Bond Ionic Bonds Valence Electrons Outer Most Electrons That Are Used In Bonding Electrons In The Highest Occupied Energy Ppt Download
Will the molecule or ion be diamagnetic or paramagnetic.

. Answer 1 of 5. Bond order 3 Part B. Bond order 1 Part E.
The manifold of states lookes like. Express your answer using two significant figures. There is a total of 11 electrons so you have something like N-O as your molecule.
Bond Order in Molecular Orbital Theory. For instance the bond order of carbon dioxide and methane is 4 which can easily be discerned by examining their Lewis structures. The following table is.
2Determine the bond order in a molecule or ion with 13 valence electrons. A molecule or ion with four electrons in bonding orbitals and two electrons in an antibonding orbital has a bond order of. Procedure to draw the molecular orbital diagram of CN.
1Determine the bond order in a molecule or ion with 10 valence electrons. Notice how the magnitude of electrons shared between each pair adequately fills the valence. Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons.
Clearly carbon has 4 valence electrons and nitrogen has 5. Find the valence electron of each atom in the CN molecule. This is the best answer based on feedback and ratings.
Bond Order 1bonding electrons1antibonding e 2 0 B o n d O r d e r 1 b o n d i n g e l e c t r o n s 1 a n t i b o n d i n g e 2 0. Completing the diagram for N 2 in the same manner as demonstrated previously we find that the 10 valence electrons result in 8 bonding electrons and 2 antibonding electrons for a predicted bond order of 3 a triple bond. DIAMAGNETIC 10 valence electrons and 14 Valence electrons PARAMAGNETIC 12 Valence electrons and 13 valence electrons.
If you want to calculate bond order of different moleculethen follow some steps. You are talking about a molecular orbital manifold of states that is pretty predictable for such a molecule. Calculate the sum of electron of given molecules.
Up to 256 cash back As a result the molecule is a paramagnetic with two unpaired electrons. The bond order of O 2 molecule. The bond order of this ion is 3.
Bond order 2 Part C. Valence Bond Theory 76. Bond order 15 Part D.
Also using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. The above formula verifies breaking the H 2 bond which in this case gives a bond order of zero. C O 2 2 ion has 10 valence electrons and the el.
The number of lines or more precisely the number of chemical bonds that comprise a molecule is called its bond order. 25 15 3 1 2 arrow_forward arrow_back_ios. 3Determine the bond order in a molecule or ion with 14 valence electrons.

Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride

Unshared Pair Easy Science Easy Science Science Student Science Rules

Valence Electrons Electrons In The Highest Energy Level Only S P Ppt Download
8 Atoms Tend To Form Bonds Until Their Valence Electron Shell Is Filled Labxchange

Chemical Bonding And Molecular Structure Cbse Notes For Class 11 Chemistry 10 Chemistry Molecular Molecular Structure
8 Atoms Tend To Form Bonds Until Their Valence Electron Shell Is Filled Labxchange
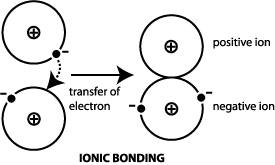
Ces Information Guide Materials Science Engineering

1 Bond And Lone Pairs Valence Electrons Are Distributed As Shared Or Bond Pairs And Unshared Or Lone Pairs Valence Electrons Are Distributed As Shared Ppt Download
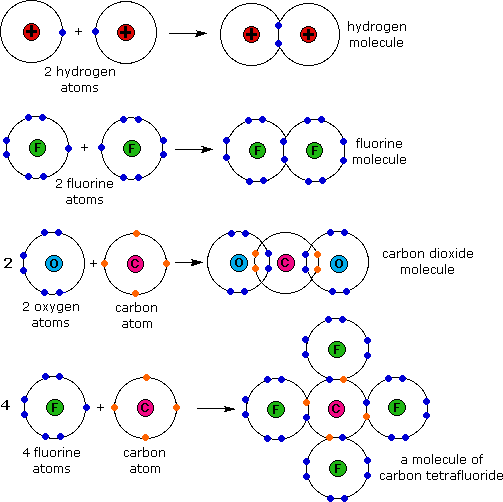
Electron Configurations The Periodic Table
8 Atoms Tend To Form Bonds Until Their Valence Electron Shell Is Filled Labxchange

Wingardium Leviosa Study Inspiration Notes Inspiration Study Motivation

Ionic And Covalent Bonds Color By Number Happy Valentine S Day Covalent Bonding Ionic And Covalent Bonds Happy Valentines Day

Xeo3 Lewis Structure Xenon Trioxide Lewis Molecules Electrons
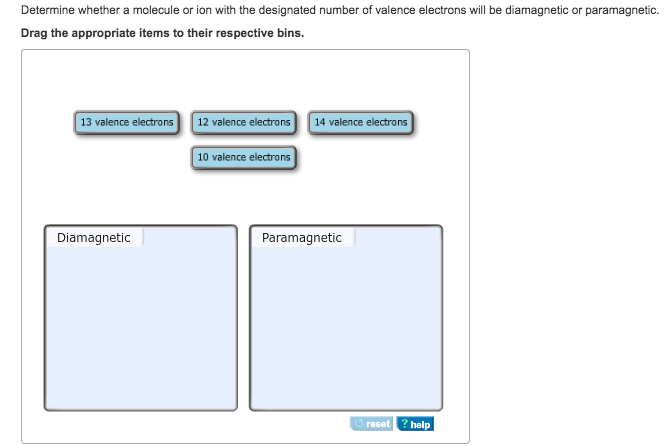
Solved 1 Determine The Bond Order In A Molecule Or Ion With Chegg Com

Unit 4 Introduction To Bonding Valence Electrons Ionic Bonding Ppt Download
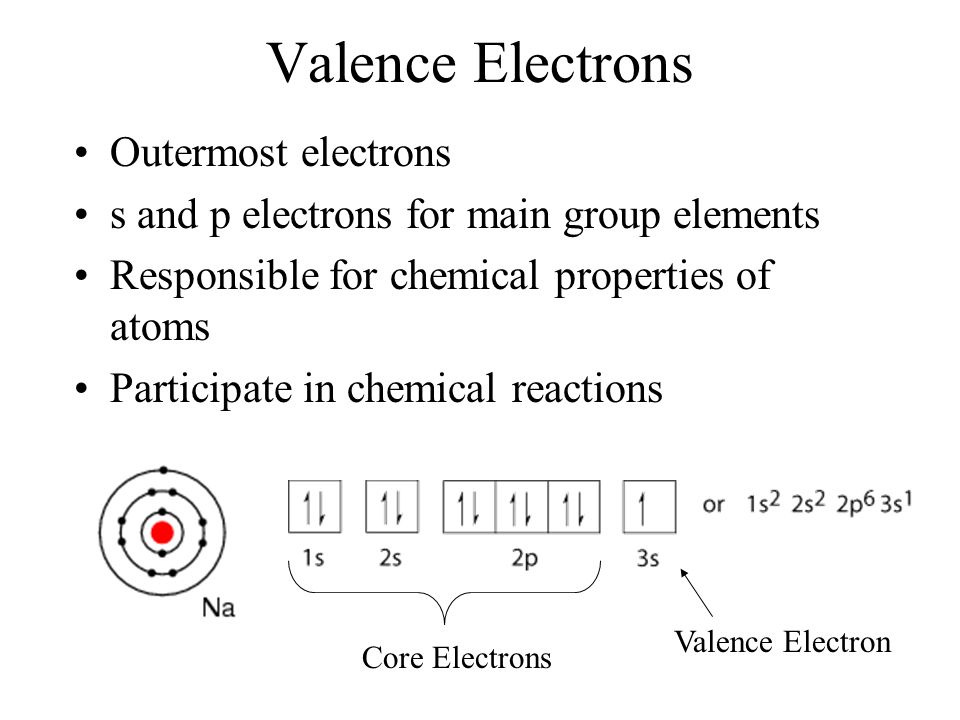
Chapters 4 5 Chemical Bonding Valence Electrons Outermost Electrons S And P Electrons For Main Group Elements Responsible For Chemical Properties Of Ppt Download

Examples Of Polar And Nonpolar Molecules Chemical Bond Chemistry Covalent Bonding

